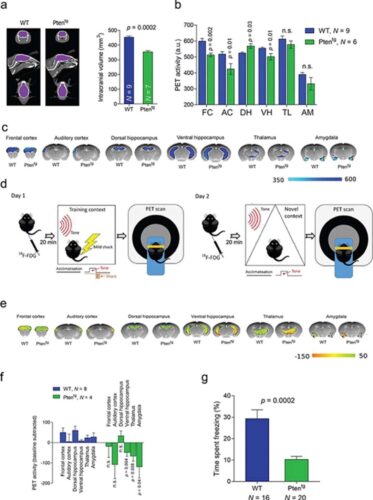
Phosphatase and tensin homolog on chromosome 10 (PTEN) is a tumor suppressor and autism-associated gene that exerts an important influence over neuronal structure and function during development. In addition, it participates in synaptic plasticity processes in adulthood. As an attempt to assess synaptic and developmental mechanisms by which PTEN can modulate cognitive function, we studied the consequences of 2 different genetic manipulations in mice: presence of additional genomic copies of the Pten gene (Ptentg) and knock-in of a truncated Pten gene lacking its PDZ motif (Pten-ΔPDZ), which is required for interaction with synaptic proteins. Ptentg mice exhibit substantial microcephaly, structural hypoconnectivity, enhanced synaptic depression at cortico-amygdala synapses, reduced anxiety, and intensified social interactions. In contrast, Pten-ΔPDZ mice have a much more restricted phenotype, with normal synaptic connectivity, but impaired synaptic depression at cortico-amygdala synapses and virtually abolished social interactions. These results suggest that synaptic actions of PTEN in the amygdala contribute to specific behavioral traits, such as sociability. Also, PTEN appears to function as a bidirectional rheostat in the amygdala: reduction in PTEN activity at synapses is associated with less sociability, whereas enhanced PTEN activity accompanies hypersocial behavior.